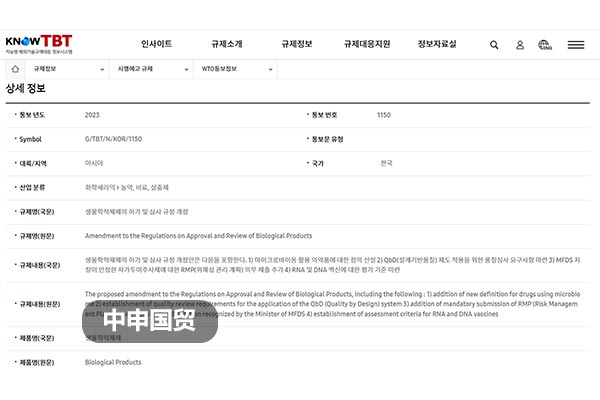
South Korea Revises Licensing and Review Regulations for Biological Products
On June 26, 2023, the Ministry of Food and Drug Safety (MFDS) of South Korea submitted Notification G/TBT/N/KOR/1150 to the WTO, proposing revisions to the Regulations on Licensing and Review of Biological Products, etc. to enhance domestic biological product safety. The revisions include definitions for new terms such as live bacterial preparations, quality audit requirements for Quality by Design (QbD) systems, expansion of the Scientific Citation Index scope for pharmacological and clinical trial data, changes to stability test data submission requirements for manufacturing method modifications, mandatory submission procedures for Risk Management Plans (RMP) for self-injection products approved by MFDS, and establishment of evaluation standards for RNA and DNA vaccines. Given that China is the worlds second-largest biological product market, these revisions have significant implications for Chinese exporters, who must closely monitor and adapt to these changes.











 PSB Record: Shanghai No.31011502009912
PSB Record: Shanghai No.31011502009912



